Recent Publications
Summaries of recently published work
Response Differences in Astrocytes Across the Lifespan
Microglia and astrocytes are cells in the brain that are actively involved in neural development and may contribute to developmental differences in the brain’s ability to “bounce back” from injury. A study conducted by graduate student Andrew J. Riquier and Dr. Suzanne Sollars showed that when early life damage occurs in rats, the microglia response is smaller and the astrocyte response larger, than when damage occurs in adulthood. To test the interplay between these two types of glia, they utilized a novel method to selectively remove microglia from the brain following neural injury in developing and adult rats. They found that when microglia were removed in adults, the astrocyte response to damage was prevented, suggesting microglia are needed for an astrocyte response to occur. However, a larger astrocyte response to neural injury during early development occurred regardless of microglia presence, suggesting the presence of a currently unknown “trigger” for astrocytes that occurs during early development but not in adulthood. These results further elucidate how microglia and astrocytes differ across development, providing insight into the mechanisms underlying neuroplasticity.
Read the complete study.
Neural Injury During Development Results in Lifelong Deficits
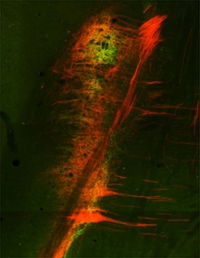
Compared to adults, developing animals experience much poorer outcomes following nerve injury. When the gustatory chorda tympani nerve is cut in rats 10 old or younger, the nerve fails to regenerate. This lack of nerve regeneration is unique among peripheral nerves and the causes of this impairment are unknown. In a recent study, we assessed the impact of early chorda tympani cuts on neuronal survival and brain pathways. We found that when chorda tympani cuts were performed in developing or adult rats, fewer neurons survived in young rats. The percentage of neurons that survive early nerve damage is similar to those in other systems where regeneration occurs, suggesting that the absence of nerve targets after injury is responsible for the lack of regeneration. The area where the chorda tympani nerve synapses onto the brain (the chorda tympani terminal field) is much sparser after cutting the nerve at five or ten days of age, but some nerve fibers are spared. Nerve integrity, therefore, does not appear necessary for central nerve maintenance. The organization of adjacent gustatory nerve terminal fields is altered when the chorda tympani is severed at five days of age, but not when it is cut at ten days of age. This finding highlights the plasticity of the gustatory system, particularly at early developmental stages. Overall, our results highlight the extreme vulnerability and plasticity of this unique system during development.
Read the complete study.
Differences in Microglia Across Development
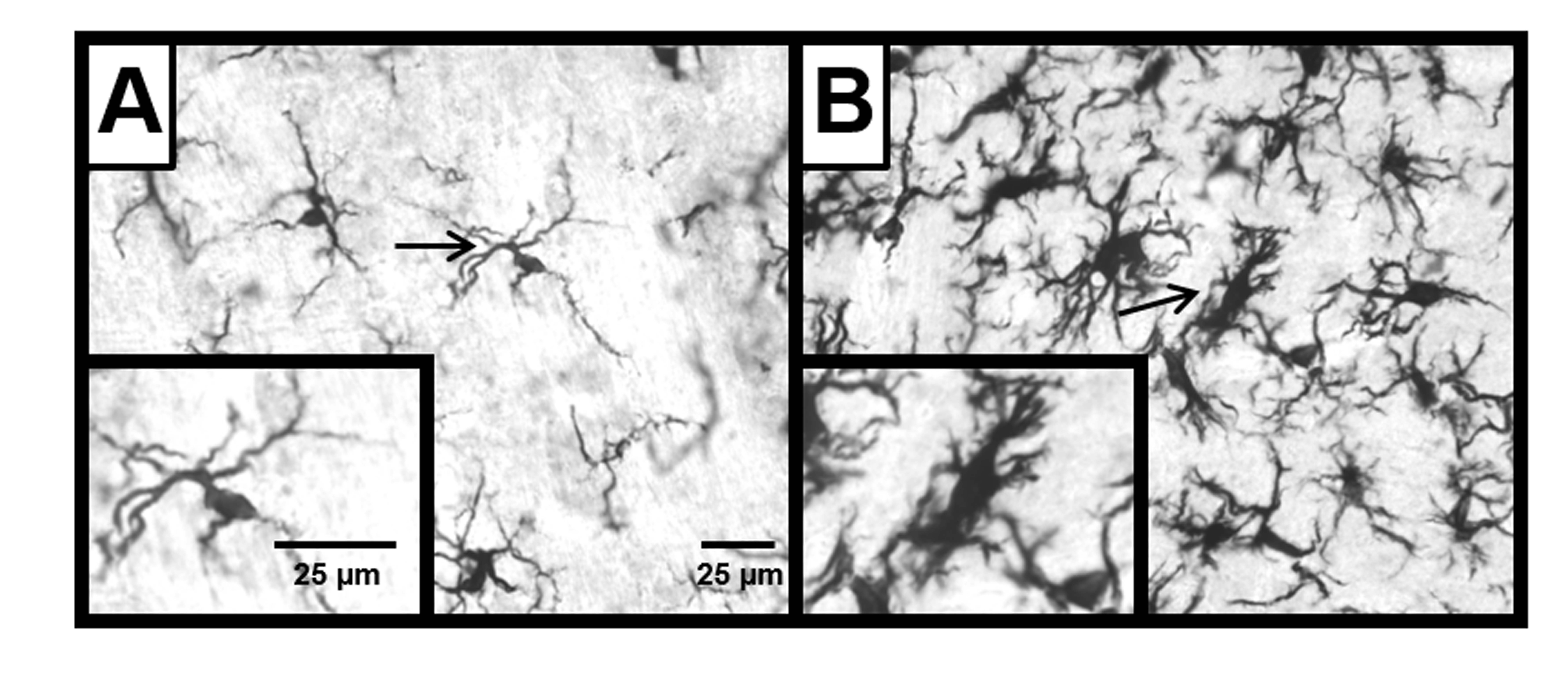
Our brains have an incredible capacity to change. Researchers in the Sensory System Development Lab use the taste system to study this adaptability both during development and following neural injury. The taste system in the brain undergoes dramatic restructuring over the course of development. Additionally, when a taste nerve, the chorda tympani, is injured in adult rats, full regeneration occurs within several weeks. However, when an identical injury occurs in young rats, permanent full recovery never occurs. A prominent facilitator of neural restructuring and adaptability are microglia, immune cells of the brain that play pivotal roles during normal development and following injury. Graduate student Andrew J. Riquier and advisor Dr. Suzanne I. Sollars recently published a paper in Neuroscience examining the role of microglia in the developing and injured rat taste system. They found that microglia number is greatest in the taste system during key periods of developmental restructuring, then decreases into adulthood, highlighting a potential role for microglia in the maturation of the taste system. Furthermore, following chorda tympani injury, microglia increase in number around the chorda tympani fibers in the brain. However, while microglia numbers increased modestly following injury early in life, adult injury induced a nearly twofold increase in microglia number. These findings demonstrate a developmental difference in how microglia respond to chorda tympani injury, which mirrors age-related differences in recovery outcomes.
Read the full paper.
Male and Female Brains Function Differently

Researchers in neuroscience often exclusively use one sex (typically males) and assume that the subject’s sex is irrelevant. Given the importance of animal research in developing treatment options for men and women, the National Institutes of Health recently implemented a new guideline for biomedical researchers to include both male and female animals in their studies. Graduate student Louis Martin and his advisor Dr. Suzanne Sollars were invited to write a review paper for a special issue of the Journal of Neuroscience Research entitled An Issue Whose Time Has Come: Sex/Gender Influences on Nervous System Function. Their review highlights human and animal research showing differences between males and females in taste system function and taste-related behavior. They also contributed novel experimental data, demonstrating that in male and female rats, taste information is processed differently even before it reaches the brain. This paper and the rest of the special issue clearly show that sex differences exist throughout the entire nervous system, and that it is important that both males and females are utilized in neuroscience research.
Read the full paper.
Frequent, Early Exposure to Spice Leads to Smaller Taste Buds in Adulthood

Love it or hate it, you are probably familiar with the effects of capsaicin, the chemical compound that gives chili peppers their characteristic spicy burn. What you may not know is that this chemical is also a mild neurotoxin, and a recently published study by researchers at UNO examined effects to the tongue caused by frequent capsaicin consumption. Graduate students Jacquelyn Omelian, Kaeli Samson and advisor Dr. Suzanne Sollars performed a series of experiments in which they fed either young or adult rats a sugar solution containing a low dose of capsaicin every day, for 40 days. The amount of capsaicin used was very mild (5ppm) compared to what humans typically consume (e.g., the average jalapeno clocks in at around 150ppm). Adult rats that drank the capsaicin had no changes in the size of their taste buds, however rats that grew up drinking capsaicin had smaller taste buds than controls when examined in adulthood. Furthermore, the mild amount of capsaicin used in the experiments did not cause inflammation in the tongue, as has been observed with higher levels of capsaicin. Overall, these findings suggest that exposure to even a mild toxin can lead to long-term changes in the taste system. It is not yet known whether similar changes may occur in humans, and future studies will look for differences in people who eat a lot of spicy food, particularly as children.
Read the full paper.
Loss of the Trigeminal Nerve Leads to Changes in Taste System, Particularly in Younger Animals

Early Nerve Injury May Permanently Alter Sensory Input to the Brain
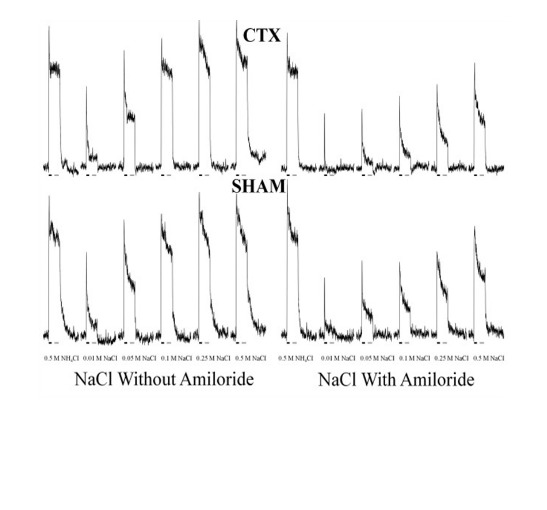
While adults have difficulty recovering following injury to the brain, children show remarkable restoration of neural function after similar types of damage. The opposite is often true in the peripheral nervous system, however. For instance, the nerve that relays taste information from the front of the tongue to the brain, the chorda tympani nerve (CT), regenerates several weeks after it is surgically cut (CTX) in adult rats. Consequently, these animals experience an almost complete recovery of sensory function and taste-guided behavior. On the other hand, previous studies from our lab have shown that when CTX occurs 10 days after birth, the nerve fails to regenerate, taste buds innervated by the CT permanently disappear, and these rats gain abnormal taste preferences. The current study was performed to understand how the physiology of the taste system is altered after CTX in young rats. To test this, electrophysiological responses following taste stimulation were recorded in the nerve that innervates taste buds on the back of the tongue, the glossopharyngeal nerve (GL), two months after cutting the CT in 10-day-old rats. Additionally, taste responses from the CT were recorded one year after the contralateral CT was sectioned during this early developmental period. Rats with CTX had lower neural responses when sodium chloride (NaCl) solutions were applied to the tongue compared to control animals. This result shows that there may be a permanent decrease in receptors for sodium salts on the tongue after early CTX – even on areas of the tongue where the nerve is not present. Thus, there are likely circulating factors (such as neurotrophins or immune components) that affect receptors across the tongue when an injury occurs. Unlike the results of this study in neonates, adult CTX does not alter nerve responses, showing again the greater susceptibility of the developing taste system to injury. This suggests that developing animals are more susceptible to impaired function after neural injury. CT injury is not a rare occurrence in children; it can happen as a result of ear surgery or ear infections. The results of this study provide insight into potential mechanisms behind long-term taste alterations that can occur after such CT injury.
Read the full paper.